AI for Life Science
Custom AI Solutions for Life Sciences
Custom AI Solutions for Life Sciences
BlackCat Bio partners with pharma and life sciences teams to design and deploy custom AI solutions that enhance productivity, automate trial design, and ensure statistical and regulatory rigor from the start. Our first product automates the generation of protocols and Statistical Analysis Plans (SAPs), checking for statistical validity, and benchmarking designs against regulatory precedent.
BlackCat Bio partners with pharma and life sciences teams to design and deploy custom AI solutions that enhance productivity, automate trial design, and ensure statistical and regulatory rigor from the start. Our first product automates the generation of protocols and Statistical Analysis Plans (SAPs), checking for statistical validity, and benchmarking designs against regulatory precedent.


products
AI Powered Software Solutions
AI Powered Software Solutions
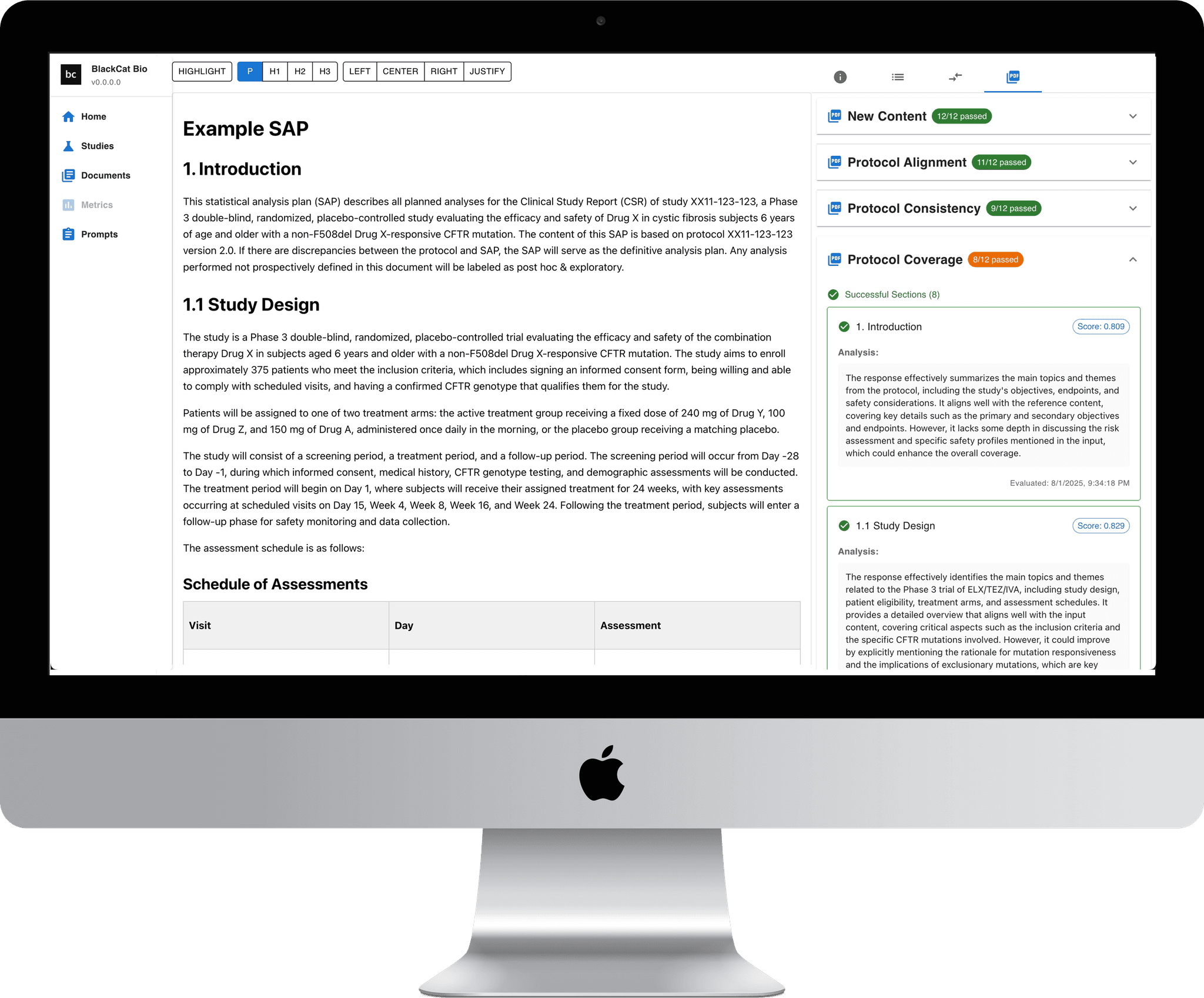
SAP-Assistant
SAP-Assistant was designed to streamline the creation and review of Statistical Analysis Plans (SAPs) for clinical trials. Leveraging a proprietary database of statistical methodologies from previously approved drug programs, it provides statisticians with intelligent drafting assistance, automated consistency checks, and compliance-focused recommendations. By combining AI with proven regulatory knowledge, SAP-Assistant reduces time, improves accuracy, and ensures alignment with industry and regulatory standards.
SAP-Assistant
SAP-Assistant was designed to streamline the creation and review of Statistical Analysis Plans (SAPs) for clinical trials. Leveraging a proprietary database of statistical methodologies from previously approved drug programs, it provides statisticians with intelligent drafting assistance, automated consistency checks, and compliance-focused recommendations. By combining AI with proven regulatory knowledge, SAP-Assistant reduces time, improves accuracy, and ensures alignment with industry and regulatory standards.
SAP-Assistant
SAP-Assistant was designed to streamline the creation and review of Statistical Analysis Plans (SAPs) for clinical trials. Leveraging a proprietary database of statistical methodologies from previously approved drug programs, it provides statisticians with intelligent drafting assistance, automated consistency checks, and compliance-focused recommendations. By combining AI with proven regulatory knowledge, SAP-Assistant reduces time, improves accuracy, and ensures alignment with industry and regulatory standards.
TLF-Generator (coming soon)
TLF-Generator is a powerful software tool that automates the creation of tables, listings, and figures (TLF) shells while ensuring they align seamlessly with your SAP and organizational standards. It not only generates high-quality outputs but also performs automated validation against shells, catching mistakes, calculation issues, and rounding errors before they reach final review. By eliminating the need for manual number-checking, TLF-Generator frees up valuable statistical resources to focus on higher-level analysis and strategy.
TLF-Generator (coming soon)
TLF-Generator is a powerful software tool that automates the creation of tables, listings, and figures (TLF) shells while ensuring they align seamlessly with your SAP and organizational standards. It not only generates high-quality outputs but also performs automated validation against shells, catching mistakes, calculation issues, and rounding errors before they reach final review. By eliminating the need for manual number-checking, TLF-Generator frees up valuable statistical resources to focus on higher-level analysis and strategy.
TLF-Generator (coming soon)
TLF-Generator is a powerful software tool that automates the creation of tables, listings, and figures (TLF) shells while ensuring they align seamlessly with your SAP and organizational standards. It not only generates high-quality outputs but also performs automated validation against shells, catching mistakes, calculation issues, and rounding errors before they reach final review. By eliminating the need for manual number-checking, TLF-Generator frees up valuable statistical resources to focus on higher-level analysis and strategy.
Study Designer
Study Designer helps clinical researchers design trials by leveraging data from competitor drugs in the same or similar indications. It automatically identifies endpoints that the FDA has historically required for approval in that therapeutic area, ensuring the study is aligned with regulatory expectations. The app also analyzes precedent studies to recommend an appropriate trial duration, consistent with what is typically seen for the indication. By combining competitor benchmarks with regulatory insights, the app guides users toward a study design that is both scientifically rigorous and strategically positioned for regulatory success.
Study Designer
Study Designer helps clinical researchers design trials by leveraging data from competitor drugs in the same or similar indications. It automatically identifies endpoints that the FDA has historically required for approval in that therapeutic area, ensuring the study is aligned with regulatory expectations. The app also analyzes precedent studies to recommend an appropriate trial duration, consistent with what is typically seen for the indication. By combining competitor benchmarks with regulatory insights, the app guides users toward a study design that is both scientifically rigorous and strategically positioned for regulatory success.
Study Designer
Study Designer helps clinical researchers design trials by leveraging data from competitor drugs in the same or similar indications. It automatically identifies endpoints that the FDA has historically required for approval in that therapeutic area, ensuring the study is aligned with regulatory expectations. The app also analyzes precedent studies to recommend an appropriate trial duration, consistent with what is typically seen for the indication. By combining competitor benchmarks with regulatory insights, the app guides users toward a study design that is both scientifically rigorous and strategically positioned for regulatory success.
NextGen N (for Power)
NextGen N is designed to support biostatisticians in developing realistic and defensible assumptions for clinical trial sample size and power calculations. By leveraging a proprietary database of clinical trial results across therapeutic areas, the app analyzes historical trials in the same or similar indications to identify patterns in effect sizes, variability estimates, event rates, and dropout assumptions. It then generates tailored suggestions helping ensure that the inputs reflect both regulatory expectations and real-world precedent. This evidence-based guidance streamlines the planning process, reduces the risk of under- or over-estimation, and provides a transparent rationale for key design decisions.
NextGen N (for Power)
NextGen N is designed to support biostatisticians in developing realistic and defensible assumptions for clinical trial sample size and power calculations. By leveraging a proprietary database of clinical trial results across therapeutic areas, the app analyzes historical trials in the same or similar indications to identify patterns in effect sizes, variability estimates, event rates, and dropout assumptions. It then generates tailored suggestions helping ensure that the inputs reflect both regulatory expectations and real-world precedent. This evidence-based guidance streamlines the planning process, reduces the risk of under- or over-estimation, and provides a transparent rationale for key design decisions.
NextGen N (for Power)
NextGen N is designed to support biostatisticians in developing realistic and defensible assumptions for clinical trial sample size and power calculations. By leveraging a proprietary database of clinical trial results across therapeutic areas, the app analyzes historical trials in the same or similar indications to identify patterns in effect sizes, variability estimates, event rates, and dropout assumptions. It then generates tailored suggestions helping ensure that the inputs reflect both regulatory expectations and real-world precedent. This evidence-based guidance streamlines the planning process, reduces the risk of under- or over-estimation, and provides a transparent rationale for key design decisions.
Biostatistics Consulting
Personalized Expert Support
Barbara will handle all statistical tasks such as study design, sample size and power calculations, Statistical Analysis Plan (SAP) development, creation of tables/listings/figures (TLF) shells, and preparation of regulatory submission packages. With deep experience across Phase II and III clinical trials, Barbara ensures that each deliverable is scientifically rigorous, compliant with regulatory standards, and tailored to the client’s specific objectives.
Personalized Expert Support
Barbara will handle all statistical tasks such as study design, sample size and power calculations, Statistical Analysis Plan (SAP) development, creation of tables/listings/figures (TLF) shells, and preparation of regulatory submission packages. With deep experience across Phase II and III clinical trials, Barbara ensures that each deliverable is scientifically rigorous, compliant with regulatory standards, and tailored to the client’s specific objectives.
Personalized Expert Support
Barbara will handle all statistical tasks such as study design, sample size and power calculations, Statistical Analysis Plan (SAP) development, creation of tables/listings/figures (TLF) shells, and preparation of regulatory submission packages. With deep experience across Phase II and III clinical trials, Barbara ensures that each deliverable is scientifically rigorous, compliant with regulatory standards, and tailored to the client’s specific objectives.
Enhanced by Proprietary Tools
Our internally developed software solutions, including SAP-Assistant and TLF-Generator, may be used as part of this work to increase efficiency and accuracy. Any time savings or efficiencies gained from these tools are passed directly on to clients, reducing project timelines and costs without sacrificing quality. By combining personalized expertise with cutting-edge proprietary tools, we deliver results that are both reliable and cost-effective.
Enhanced by Proprietary Tools
Our internally developed software solutions, including SAP-Assistant and TLF-Generator, may be used as part of this work to increase efficiency and accuracy. Any time savings or efficiencies gained from these tools are passed directly on to clients, reducing project timelines and costs without sacrificing quality. By combining personalized expertise with cutting-edge proprietary tools, we deliver results that are both reliable and cost-effective.
Enhanced by Proprietary Tools
Our internally developed software solutions, including SAP-Assistant and TLF-Generator, may be used as part of this work to increase efficiency and accuracy. Any time savings or efficiencies gained from these tools are passed directly on to clients, reducing project timelines and costs without sacrificing quality. By combining personalized expertise with cutting-edge proprietary tools, we deliver results that are both reliable and cost-effective.
About Us
Barbara Elashoff
Barbara Elashoff
I am a former FDA statistical reviewer who has reviewed hundreds of protocols and SAPs. I've also worked in pharma and biotech for the past 20 years. I have experience across a wide variety of therapeutic areas including pulmonary, autoimmune, metabolic, cardiovascular, dermatology and analgesia. I have a proven track record of successful regulatory submissions including NDAs, PMAs, 510(k)s, and BLAs.
I am a former FDA statistical reviewer who has reviewed hundreds of protocols and SAPs. I've also worked in pharma and biotech for the past 20 years. I have experience across a wide variety of therapeutic areas including pulmonary, autoimmune, metabolic, cardiovascular, dermatology and analgesia. I have a proven track record of successful regulatory submissions including NDAs, PMAs, 510(k)s, and BLAs.
Publications
View my CV
View my CV
Former FDA Reviewer
Former FDA Reviewer
Masters from Harvard
Masters from Harvard
Experience in Pharma, Devices & MedTech
Experience in Pharma, Devices & MedTech
Former FDA Reviewer
Masters from Harvard
Experience in Pharma, Devices & MedTech
Shoham Das
Shoham Das
AI-focused software engineer and data engineering leader with 7+ years of experience delivering scalable systems across biotech, healthcare, and venture. Skilled in building full-stack applications, data infrastructure, and AI-powered tools that support scientists, clinicians, and investors—spanning high-throughput experimentation, therapeutic discovery, and portfolio strategy.
AI-focused software engineer and data engineering leader with 7+ years of experience delivering scalable systems across biotech, healthcare, and venture. Skilled in building full-stack applications, data infrastructure, and AI-powered tools that support scientists, clinicians, and investors—spanning high-throughput experimentation, therapeutic discovery, and portfolio strategy.
Github
Biotech & Healthcare Expertise
Biotech & Healthcare Expertise
Github
AI-Focused Software Engineer
AI-Focused Software Engineer
Github
AI-Focused Software Engineer
Biotech & Healthcare Expertise
What Clients Say
As a former colleague of Barbara Elashoff, I highly recommend Barbara as a biostatistician, strategist and clinical development expert. As a biostatistician, Barbara is in her element, performing all aspects of stats-related development, from early protocol design and authoring, to high quality statistical analysis plans, to providing statistical outputs and clinical study reporting. Her strong background in programming and development of creative study visualizations and outputs will be an asset to every project that she touches. As a Clinical Operations leader, I especially appreciate Barbara’s ability to explain her field and output to non-statisticians; with her effective communication style that can flex for her audience. Barbara will be an excellent choice to fill your clinical development strategic and statistical gaps! -Abby Kennedy, VP Clinical Operations
As a former colleague of Barbara Elashoff, I highly recommend Barbara as a biostatistician, strategist and clinical development expert. As a biostatistician, Barbara is in her element, performing all aspects of stats-related development, from early protocol design and authoring, to high quality statistical analysis plans, to providing statistical outputs and clinical study reporting. Her strong background in programming and development of creative study visualizations and outputs will be an asset to every project that she touches. As a Clinical Operations leader, I especially appreciate Barbara’s ability to explain her field and output to non-statisticians; with her effective communication style that can flex for her audience. Barbara will be an excellent choice to fill your clinical development strategic and statistical gaps! -Abby Kennedy, VP Clinical Operations
As a former colleague of Barbara Elashoff, I highly recommend Barbara as a biostatistician, strategist and clinical development expert. As a biostatistician, Barbara is in her element, performing all aspects of stats-related development, from early protocol design and authoring, to high quality statistical analysis plans, to providing statistical outputs and clinical study reporting. Her strong background in programming and development of creative study visualizations and outputs will be an asset to every project that she touches. As a Clinical Operations leader, I especially appreciate Barbara’s ability to explain her field and output to non-statisticians; with her effective communication style that can flex for her audience. Barbara will be an excellent choice to fill your clinical development strategic and statistical gaps! -Abby Kennedy, VP Clinical Operations
FAQs
Will my protocol or data be used to train your AI?
What security measures do you have in place?
Can the software integrate with our existing workflows?
What is your AI policy?
Does the software replace statisticians?
Do you license your software?
Will my protocol or data be used to train your AI?
What security measures do you have in place?
Can the software integrate with our existing workflows?
What is your AI policy?
Does the software replace statisticians?
Do you license your software?
Contact


